 You are entering webpages belonging to the American Association of Orthopaedic Surgeons, which includes information and resources for Association advocacy efforts and the Political Action Committee of The American Association of Orthopaedic Surgeons (OrthoPAC).
You are entering webpages belonging to the American Association of Orthopaedic Surgeons, which includes information and resources for Association advocacy efforts and the Political Action Committee of The American Association of Orthopaedic Surgeons (OrthoPAC).
Antitrust
Cleared Senate objections to legislation that would repeal the McCarran-Ferguson Act, which has protected insurers since 1945. By removing the unfair exemption, the federal government is now empowered to enforce the full range of antitrust laws against health insurance companies engaged in anticompetitive conduct, including collaboration on pricing. This legislation, called the Competitive Health Insurance Reform Act, was signed into law by the president on January 13, 2021.
Learn more about this topic in a related podcast episode.
August 2020 In-District Advocacy Event
AAOS fellows spent the month of August 2020 meeting with their congressional representatives in-person, by video call or by telephone to advocate for high-priority issues of importance to the musculoskeletal community. Although fellows are annually encouraged to meet with their representatives indistrict at this time of year—when Congress is typically in
recess—this was the first time that AAOS, in partnership with state orthopaedic societies, coordinated a nationwide campaign of this scale. Despite the many challenges presented as a result of COVID-19, the virtual nature of the event allowed for more orthopaedic voices to be heard at a time when physician leadership on healthcare policy is critical.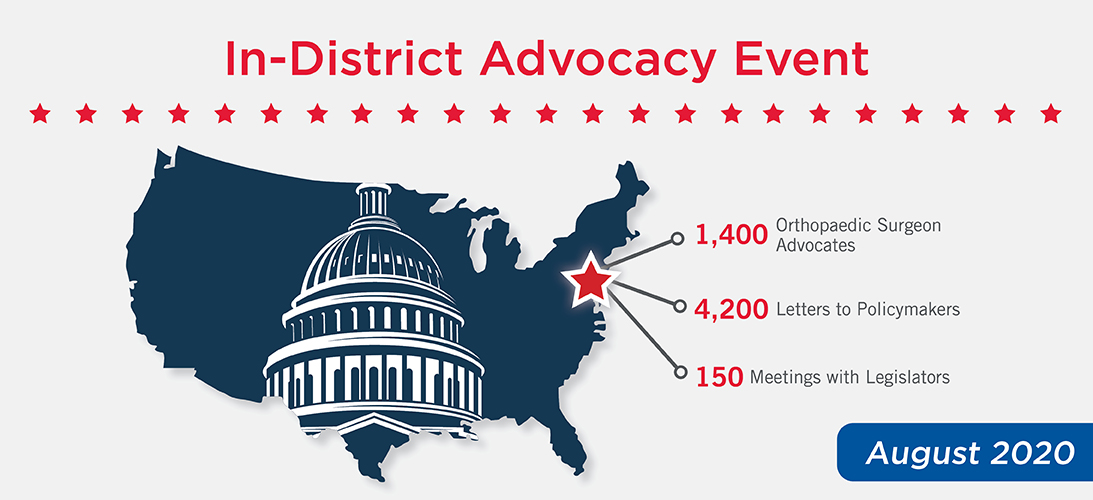
Advocates spoke with members of Congress about payment policy changes, medical liability reform, prior authorization reform, and lifting restrictions on physician-owned hospitals. In addition to the event results in the above graphic, the Orthopaedic PAC ran a parallel campaign that generated
$147,099 dollars raised from 638 contributors and 26 fundraising events.
Learn more about the event.
COVID Relief
Advocated with federal regulators, through meetings and written requests, to distribute adequate personal protective equipment for frontline COVID-19 care teams and change the eligibility definitions for receipt of Provider Relief Funds during the pandemic. These efforts led to 1,760 orthopaedic practices across the country receiving $296,967,483 in grants by July 2020.
Grassroots Activity
In 2020, AAOS saw great growth and engagement through its grassroots program in the Advocacy Action Center including the utilization of regulatory comments for the first time:
- 18,400 total grassroots messages sent – a 490%
increase from the 3,120 messages sent in 2019 - The top issues members engaged on were:
- Evaluation and Management Code Issues - 13,200 messages
- Surprise Medical Billing - 1,800 messages
- Prior Authorization - 477 messages
- AAOS sent a total of 12 grassroots alerts to the membership asking them to take action
So far in 2021, YTD AAOS members have sent 1,355 messages to Congress on the following issues:
- Prior Authorization – 694 messages
- Medicare Cuts – 508 messages
- Physician Mental Health – 81 messages
- Telehealth Services – 72 messages
The AAOS sent one grassroots alert to members in February 2021 asking them to take action on Medicare cuts.
Learn more about the grassroots program.
Medicare Cuts
Worked with Congress to mitigate Medicare cuts to specialty care slated for 2021. Language to partially prevent Medicare cuts was included in the combined $900 billion COVID-19 aid package and $1.4 trillion omnibus spending bill that was signed into law by the president on December 27, 2020.
Orthopaedic Advocacy Week Event, May 24-28, 2021
Orthopaedic Advocacy Week was created at the request of the AAOS Presidential Line. Each day of the first-ever completely virtual event utilized a tactic in AAOS’ advocacy strategy toolbox for shaping healthcare policy and delivering critical messages to lawmakers: Growing Support with Grassroots, Amplifying our Message with Social Media, Shaping Policy through Rulemaking, a Virtual Capitol Hill Day with Lawmakers, and Getting Invested with the Orthopaedic PAC. In addition to the results listed in the graphic below, 249 members participated in the event from 39 states and Puerto Rico. Importantly 109 new legislative cosponsors were generated for bills to reform prior authorization, expand telemedicine services, and prioritize physician mental health.
Learn more about the event.
Research Funding
Secured the support of 77 members of Congress for our requested $35 million for the peer-reviewed orthopaedic research program in the FY 2021 Defense Appropriations legislation. The program received $30 million, consistent with past levels of funding.
Surprise Billing
Worked with Congress to protect patients from surprise medical bills and avoid a federal benchmark for resolving out-ofnetwork billing disputes. A solution containing an independent dispute resolution process and other AAOS priorities was included in year-end legislation that was signed into law. AAOS also submitted self-initiated comments to the U.S. Department of Health and Human Services to highlight these priorities prior to rulemaking and is continuing to comment in response to new regulations that implement the law.