AAOS has launched the Biologics Dashboard, a new member benefit that recognizes the need for clarity and conciseness in the evolving field of biologics. The Biologics Dashboard synthesizes numerous sources of information into a smart tool that is easy to query with a few clicks to deepen surgical decision making in biologics.
The field of orthobiologics holds great promise for orthopaedic surgeons. It also brings some challenges such as good controls for clinical studies, conflicting data, and therefore confusion and controversy regarding best practices. Although the FDA, industry, and academia provide a wealth of data, it is difficult to assess the consensus regarding acceptable uses of biologics supported by the relevant regulatory and evidentiary information. Where there is no current consensus, the relevant regulatory and evidentiary information is equally critical for informed surgical decision making and patient consent.
The release of the Biologics Dashboard emphasizes regulatory affairs, as these have recently evolved to set boundaries for clinical use. The dashboard also contains pointers to Level I evidence in the form of pivotal randomized clinical trials that were used to support FDA approval of biologics via references to the FDA labeling. Similarly, it is a natural home for the Technology Reviews that AAOS is currently performing for multiple aspects of biologics; these include comprehensive evidentiary reviews when they are conclusive.
User experience
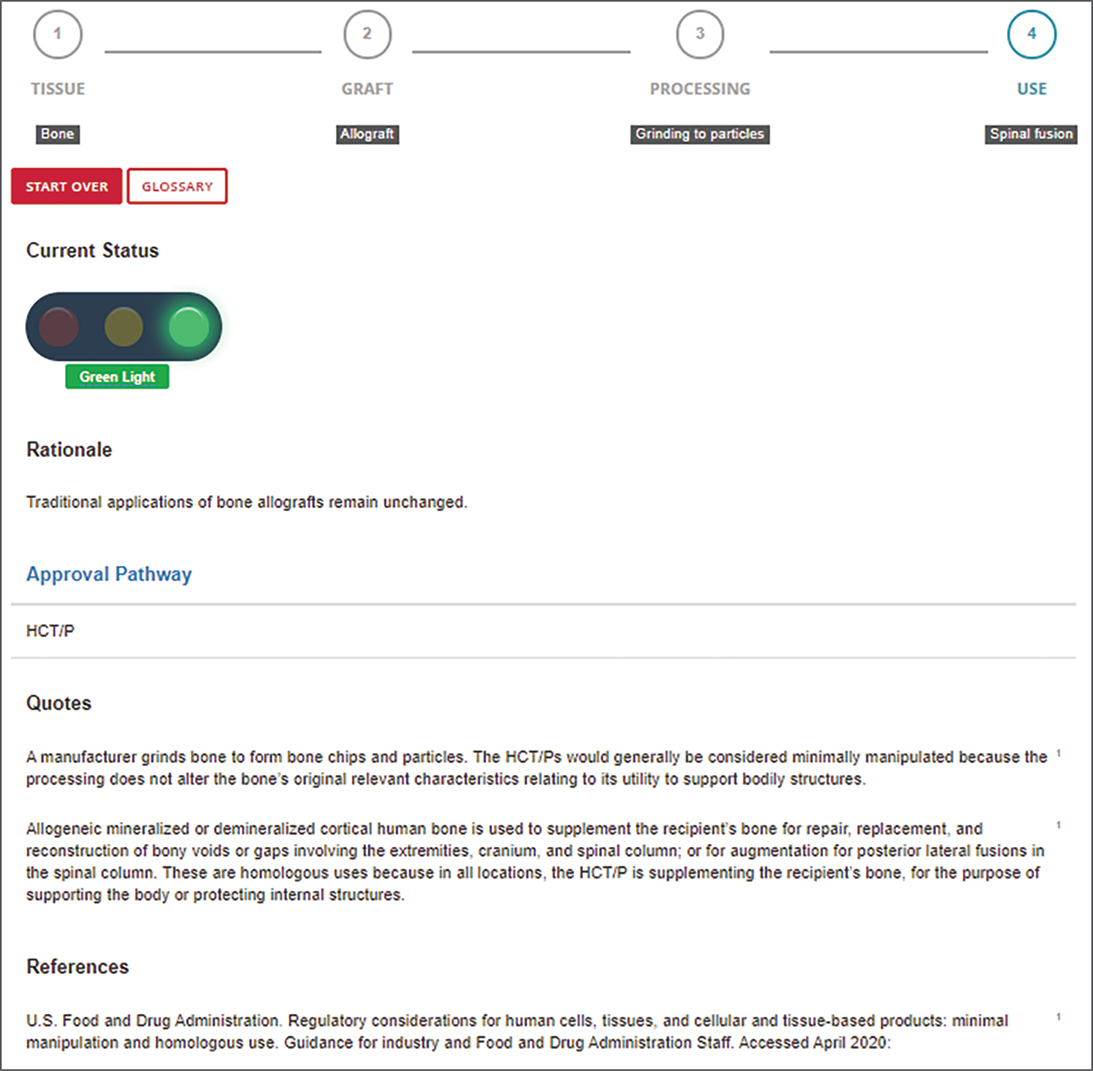
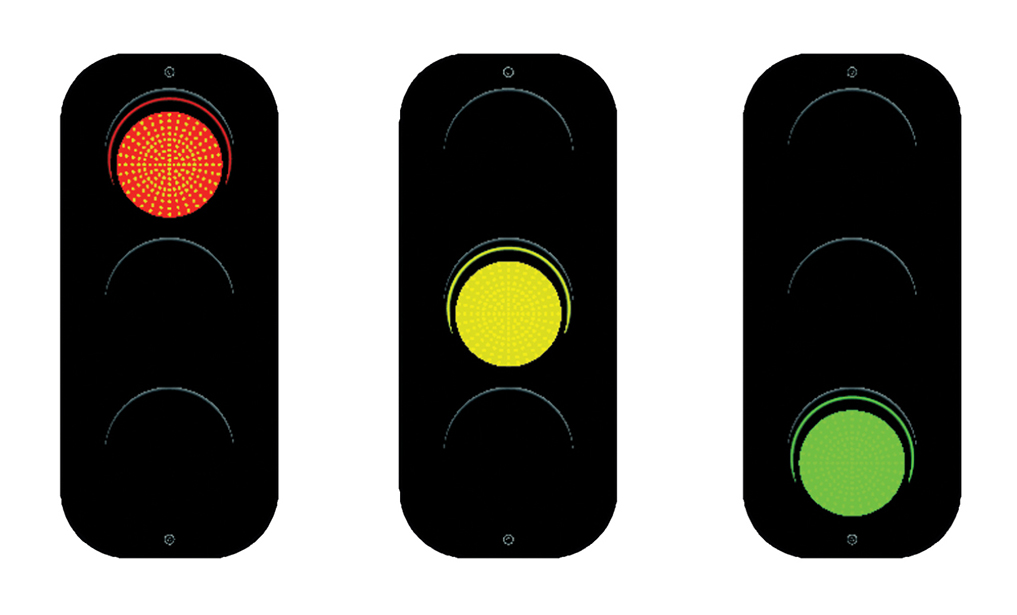
The Biologics Dashboard is intended for any surgeon who has wondered about a biologic product’s safety, efficacy, regulatory status, or evidence base. The output of the dashboard is an overall recommendation using a stoplight metaphor regarding a specific biologic product and its use: a red, yellow, or green light recommendation (Fig. 1). The intention of the stoplight metaphor is to synthesize complex information in a way that is intuitive and easy to use, while retaining scientific accuracy and providing references to the source material.
To get a stoplight recommendation, a surgeon’s query only requires knowledge of four basic characteristics of the product:
- tissue type (bone, adipose tissue, amniotic membrane, etc.)
- tissue source (autograft, allograft, etc.)
- processing (washed, ground, etc.)
- intended use (bone graft, tendon repair, etc.)
Due to the intelligent design of the user experience, these clicks require only a few seconds.
Methodology
The design philosophy of the Biologics Dashboard includes three principles. The first is that the tool should be intelligent and easy to use for practicing surgeons. The second is that it should stick close to the source material when there is a clear regulatory or evidentiary recommendation. The third is that, when there is no consensus, AAOS should efficiently synthesize the highest-quality information from all sources for the surgeon: regulatory status, pivotal trials, evidentiary reviews, etc.

The Biologics Dashboard is a product of the Devices, Biologics, and Technology Committee (DBT). To accurately identify the stoplight status of each product, DBT members balanced regulatory status, device composition, intended use, the state of published literature, and FDA guidance documents. For example, some drug device combinations with premarket approval (e.g., bone morphogenetic proteins) and biologics issued a biologic license application (autologous cartilage expansion) require Level I evidence and support from randomized, controlled trials. By contrast, some of the human cell- and tissue-based therapies and products (HCT/Ps) require evidence of proper tissue processing but have been exempted from detailed studies of safety and efficacy. Finally, which categories these products fall into remains a topic of debate, despite the FDA’s attempts to clarify it. These complexities are considered and synthesized by a panel of experts.
An example of the Biologics Dashboard is shown in Fig. 2. Understanding the definitions requires certain regulatory definitions that have been popularized by AAOS (Fig. 3). For example, manufacturers of 361 HCT/Ps typically do not need to submit any bench, animal, or clinical data regarding safety and efficacy to the FDA to be able to market the products to surgeons. Some products in that category, such as allograft cancellous bone chips used to supplement a bony defect, do not have specific vetting of safety and efficacy by the FDA but have a long track record of safety and efficacy in the orthopaedic literature. Others are introduced into the market with the vendors’ belief that they meet the 361 HCT/P criteria, but they may have ambiguous status until the FDA is successful in reacting to the product.
Conclusion
The Biologics Dashboard requires a few clicks to provide a synthesized recommendation for the use of a biologic along with the rationale for the recommendation and the evidence-based source material. With the launch of this online resource, it is the intention of the DBT to equip members with a better understanding of emerging and established biologic-based products, the evidence that has accompanied the products to market, and the various FDA approval pathways that exist for these types of products. As available evidence and federal guidance evolve and new products come on the market, the dashboard will also continue to evolve and mature to best serve AAOS members.
Access the Biologics Dashboard at www.aaos.org/biologicsdashboard.
S. Raymond Golish, MD, PhD, MBA, FAAOS, is the chief medical officer of HCA Healthcare JFK Hospital in Palm Beach, Fla., and a member of the DBT. He can be reached at ray@golish.com.
Ryan Pezold, MA, is a director within the AAOS Department of Clinical Quality and Value. He can be reached at pezold@aaos.org.
Martha M. Murray, MD, MS, FAAOS, is a professor of orthopaedics at Harvard Medical School and Boston Children’s Hospital and is a member of the DBT. She can be reached at martha.murray@childrens.harvard.edu.
David S. Jevsevar, MD, MBA, FAAOS, is the chair and associate professor of orthopaedics at Dartmouth-Hitchcock Health and chair of the DBT. He can be reached at david.s.jevsevar@hitchcock.org.