A study of patients undergoing elective outpatient foot and ankle surgery and receiving a comprehensive, opioid-free, multimodal pain-management protocol found that most patients achieved excellent pain control without significant postoperative side effects.
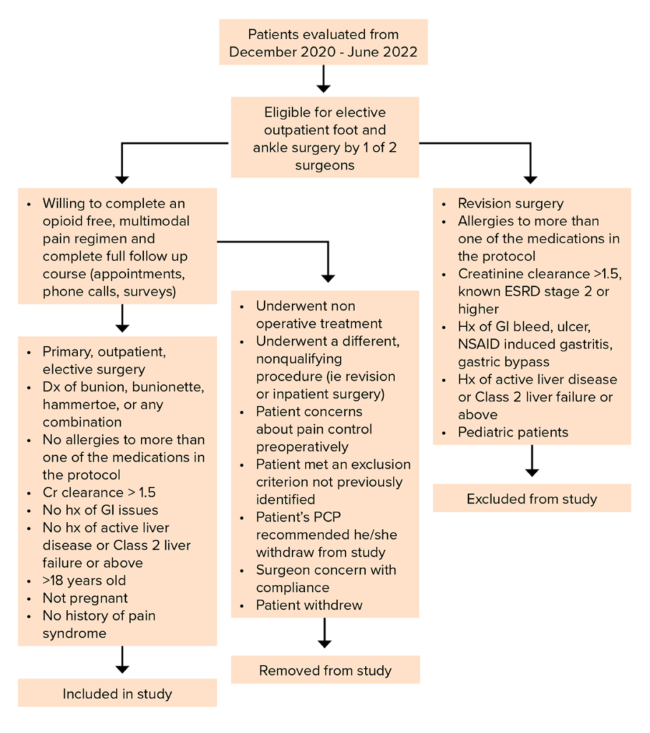
The study, presented at the AAOS 2023 Annual Meeting by J. Benjamin Jackson III, MD, MBA, of the University of South Carolina Department of Orthopedic Surgery, involved 41 patients who underwent elective, outpatient, primary foot and ankle surgery. Surgeries were one or any combination of bunion or bunionette surgery or hammertoe surgery performed by one of two fellowship-trained orthopaedic foot and ankle surgeons at a single level 1 tertiary referral center. Patient exclusion, enrollment, and withdrawal criteria are shown in Fig. 1.
No narcotics were initially prescribed during the postoperative period; however, if a patient was still in pain, rescue narcotics could be prescribed on an individual basis. All data were collected in a prospective manner and included pain visual analog scale (VAS), medication side effects, medication requirements, overall patient satisfaction, and trips to the ED or urgent care. This information was gathered via a phone call on postoperative days (PODs) 1, 3, and 8. Those same data points were collected again at the two-week follow-up, in addition to patients’ overall satisfaction. Overall, patients’ self-rated pain levels were followed for 12 weeks, and their clinical courses were followed radiographically for surgical complications for six months. Data were collected postoperatively until radiographic evidence of bony healing or until revision surgery.
The multimodal treatment protocol included oral pregabalin, acetaminophen, ketorolac tromethamine (for three days), and cyclobenzaprine to begin POD 0. Meloxicam was given on POD 4 once ketorolac tromethamine was completed.
Among the 41 patients who enrolled and completed the full follow-up, the mean age was 55 years (range, 18–81). There were 36 females and five males. The average follow-up was 11.6 weeks. On PODs 1, 3, and 8, the average VAS pain scores were 4.16, 2.59, and 1.51 out of 10, respectively. At two-week follow-up, average VAS pain score was 1.39 out of 10; patients stated on average that they expected their average pain to be a 3.51 out of 10.
Ten patients had had previous bunion surgery on the contralateral side. Nine of them stated they were in less pain or the same amount of pain with the current protocol compared with the protocol from their previous surgery. Five patients had had previous foot and ankle surgery, four of whom were in the same amount of pain or less pain with the current protocol compared to the protocol with their previous surgery.
None of the patients required a visit to the emergency department. However, seven patients (17 percent) required rescue narcotic pain medication within the first two weeks. At the POD 1 phone call, seven patients needed Norco or oxycodone for breakthrough pain. Two patients had a prescription called in; the others were taking “leftover” pain medication. By the POD 3 phone call, only three patients continued to take any narcotic pain medications; by the POD 8 phone call, only two patients were taking any narcotic pain medications. Interestingly, the two patients who were prescribed rescue narcotics were the same two patients who were still taking narcotic pain medication at POD 8. At the two-week follow-up appointment, no one was taking narcotic pain medications.
The researchers conducted statistical analysis of the Patient-Reported Outcomes Measurement Information System (PROMIS) questionnaires for physical function, pain interference, and mobility. For physical function, the 12-week follow-up measure had a mean that was significantly larger than the two-week follow-up (P <0.0001) and the six-week follow-up (P <0.0001). For pain interference, the 6-week follow-up measure had a mean that was significantly smaller than the two-week follow-up (P=0.0010) and the 12-week follow-up (P <0.0001). For mobility, the 12-week follow-up measure had a mean that was significantly larger than the two-week follow up (P <0.0001) and the six-week follow-up (P <0.0001). Overall, 88 percent (36 patients) were satisfied with their pain control, 81 percent (33 patients) would follow the same pain management again, and 85 percent (35 patients) would recommend this pain management regimen to a family member or friend.
Dr. Jackson noted that he and his colleagues undertook the investigation after a preliminary study that examined the number of narcotics that patients used after bunion surgery. “We noted that number was around 24 narcotics tabs,” he said. “Given that number and the opioid crisis, along with their associated side effects, we sought to improve patient care by devising an evidence group of medications from other subspecialities that we believed would work for foot and ankle patients.” He noted that the findings of this study could possibly be applied to pain management for procedures involving midfoot, hindfoot, ankle, and/or nonelective cases such as fractures.
Dr. Jackson’s coauthors of “Opiate-free Multimodal Pain Pathway in Elective Foot and Ankle Surgery: A Prospective Study” are Rachel Glenn, MD; Daniel Ross, MD; and Tyler Gonzalez, MD, MBA.
Terry Stanton is the senior medical writer for AAOS Now. He can be reached at tstanton@aaos.org.